Think of the DNA found in each of your cells as a vast fragile library with the entire instruction manual on how to put you together and how to keep you functioning. Suppose now, that we make a disaster, a double strand break, and cut the two ends of the DNA ladder completely in half. It is a disastrous event in a cell potentially resulting in loss of important genetic information, unwarranted cell growth (cancer) or even cell death. Luckily, we house within our cells a squad of magnificent, molecular-level librarians and maintenance crews. The most precise and sophisticated tool in their arsenal is a process called homologous recombination. This is not some sloppy fix-up, it is a high-fidelity, photocopy-and-paste system that employs a good sister copy of DNA as a model in order to reconstitute the damaged sequence exactly. Understanding homologous recombination is key to unlocking mysteries in genetics, developing new cancer treatments, and advancing revolutionary gene-editing technologies like CRISPR. The part it plays in preserving our inherited integrity cannot be overemphasized.
What Is the Fundamental Mechanism of Homologous Recombination?
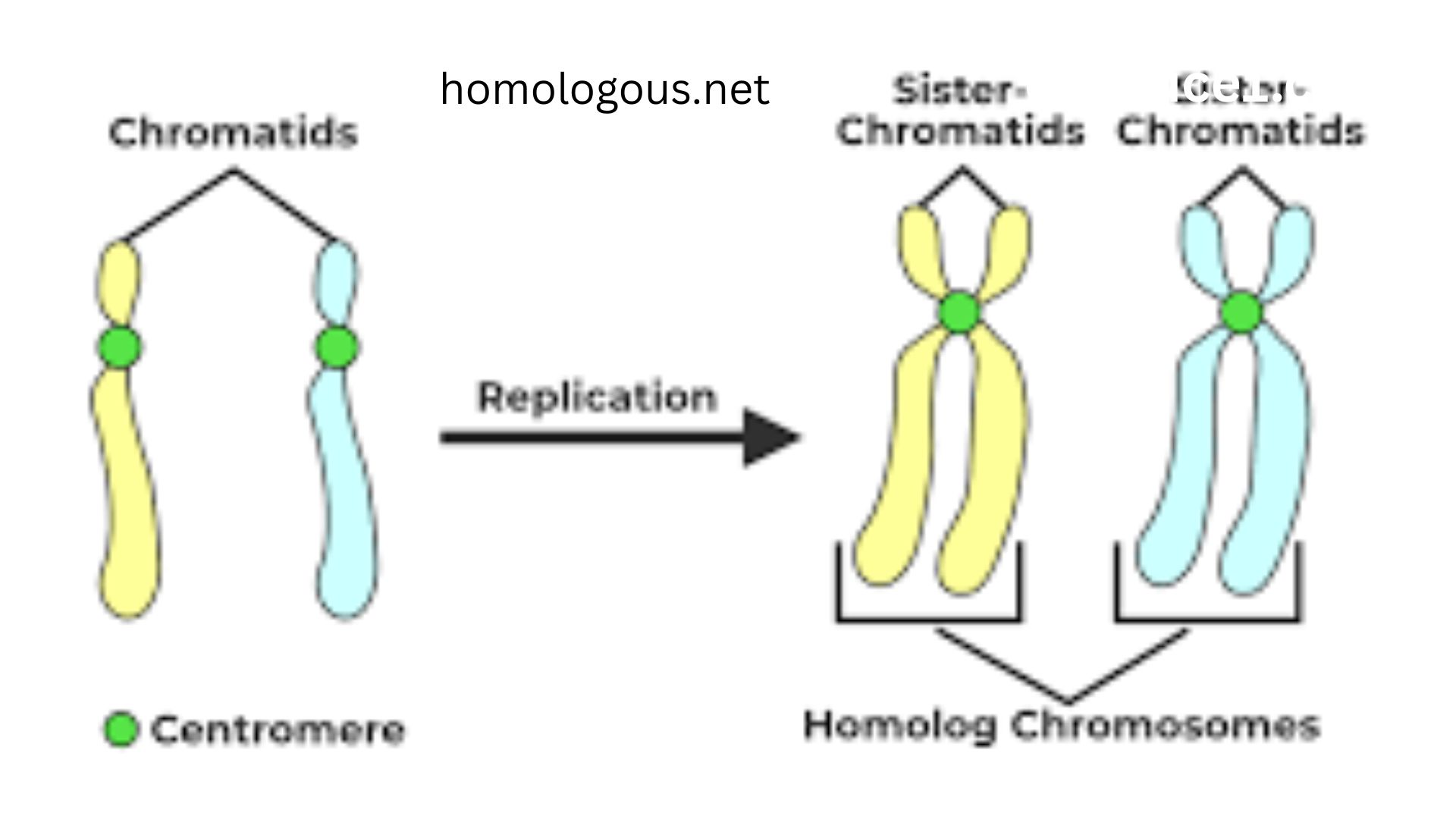
At its core, homologous recombination is a DNA repair process that mends double-strand breaks by using a nearly identical DNA sequence as a template. Homologous refers in a literal sense to being in the same relation, relative / position or structure. The use of a sister chromatid (of dividing cells) or a homologous chromosome (of the other parent) as a genetic blueprint in the assembly of perfect repair can be interpreted as in this context. It is an intricate choreography of enzymes and proteins, and it is performed with magnificent accuracy. It starts with identifying the break, followed by a step referred to as resection whereby the two ends are chewed out to the formation of single-stranded tails of DNA. One of such tails will then invade the intact DNA duplex, which is homologous to it and it will anneal with its complementary DNA duplex. This creates a scaffold whereby the cell-based machinery is able to replicate the missing portion of information in the template and then incorporate it back to the broken chromosome in a manner that it reinstates the initial genetic code defect-free. It is due to this high fidelity that distinguishes it with the other, more erroneous repair pathways.
How Does Homologous Recombination Differ From Other DNA Repair Pathways?
A set of various tools to respond to different types of DNA damage exist in the toolbox of cells. Comparing homologous recombination to its alternatives highlights its unique role and importance. There are two other main pathways in which double-strand breaks are addressed: the Non-Homologous End Joining (NHEJ) and the Microhomology-Mediated End Joining (MMEJ).
NHEJ is the hurried and sloppy repair used by the cell. It just re-ligates the two broken ends to each other, and usually produces small insertion/deletions (indels) at the junction site. Although rapid, it is very mutagenic. A similar method is MMEJ, which uses minute microhomologus sequences (several base particles) to direct the fusion, although this joining is relatively imprecise still. Homologous recombination, in contrast, is the slow, meticulous, and accurate method. It needs to have a template and the primary stage of its activity is the S stage and the G2 stage of the cell cycle when a sister chromatid exists. The decision in choosing the two different pathways is a calculated risk to the cell: speed or accuracy.
Why Is Homologous Recombination Critical for Meiosis and Genetic Diversity?
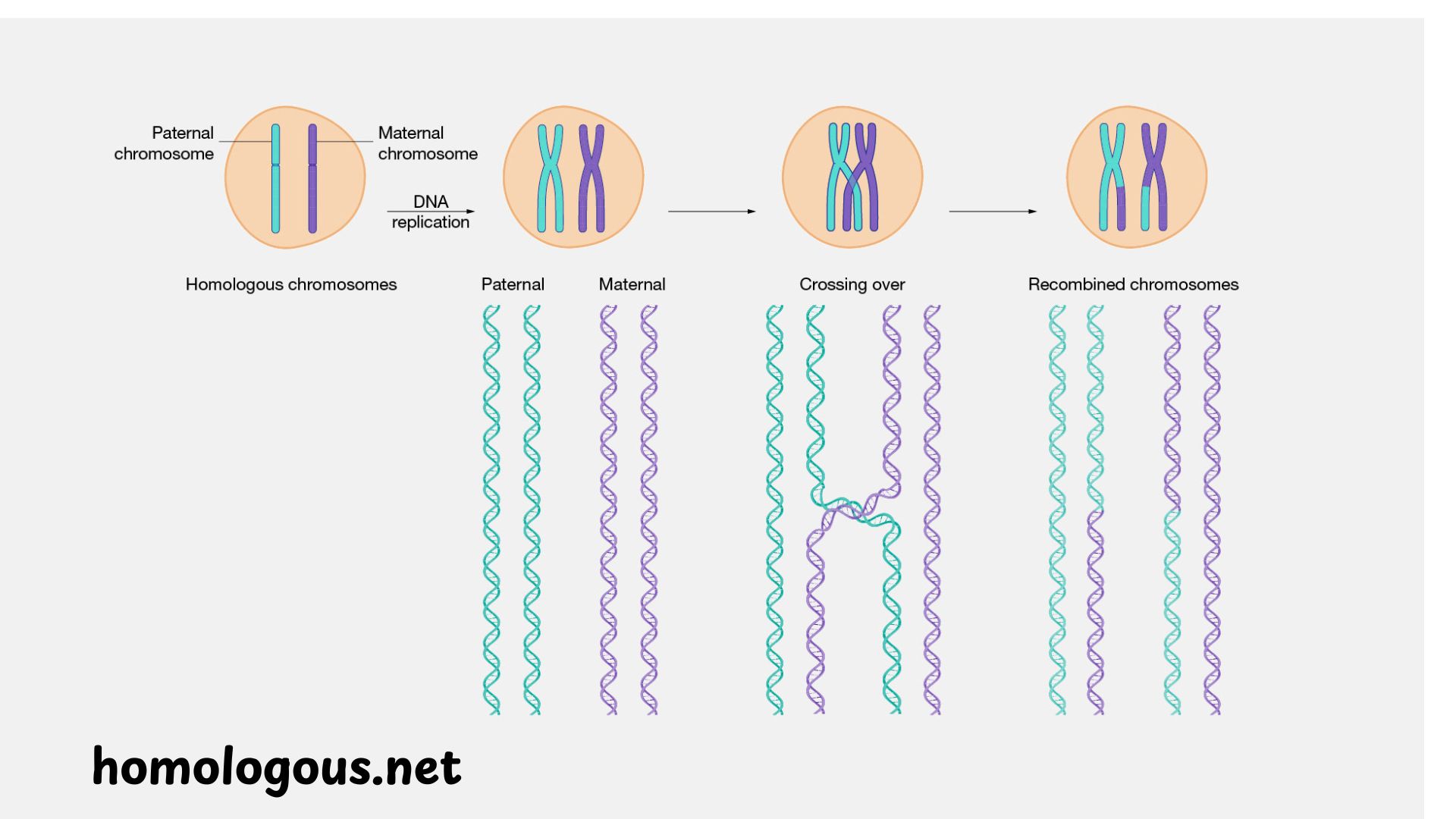
While its role in repair is vital, homologous recombination has a second, equally crucial function: it is the engine of genetic diversity in sexual reproduction. The specialized process of cell division that produces the cell products of reproduction (sperm and eggs) in a process called meiosis, induction of double-strand breaks is actually intended by the cell. The repair of these breaks via the homologous recombination pathway between paternal and maternal chromosomes is what causes “crossing over,” or genetic recombination.
This is a tinkering with the genetic deck. Rather than just retaining fully intact chromosomes as were given to them by their parents, meiosis developsВ new chromosomes which are a mosaic or distinct combination of both. Recombination makes every gamete, and consequentially every offspring, to be genetically different. It is a potent engine of evolution, thus enabling populations to respond to changing environment. Without the precise control of homologous recombination during meiosis, chromosomes could fail to separate correctly, leading to aneuploidy (an abnormal number of chromosomes), which is a leading cause of miscarriage and developmental disorders like Down syndrome.
What Happens When Homologous Recombination Fails?
When the intricate machinery of homologous recombination breaks down, the consequences are severe. Defects in the genes coding HR proteins, most notably the Bradford Hill tumor suppressor genes BRCA1 and BRCA2, disable a cell in its high-fidelity DNA repair. The cells are subsequently compelled to use inaccurate alternative pathways (such as NHEJ) to survive.
This cancer-related genomic instability is characteristic of cancer. With each division, an uncorrected mutation increases and the process propagates itself, changing a healthy cell into a cancerous cell. That is why patients with an inherited form of mutation of BRCA genes possess a disproportionately high risk to be affected by breast, ovarian, prostate, and other types of cancer during life. They are devoid of a basic custodian of the genome in their cells. This weakness is however tapped into as a strong anticancer drug approach termed as synthetic lethality. Certain pathways, such as a damaged HR pathway in a cancer cell, introduce drugs known as PARP inhibitors; these drugs are made to specifically kill cells that already have a defective HR pathway, leaving healthy cells unharmed. This is a prime example of how understanding a basic biological process like homologous recombination can directly lead to life-saving clinical therapies.
How Is Homologous Recombination Used in Genetic Engineering?
The principles of homologous recombination have been harnessed by scientists to perform targeted genetic engineering long before CRISPR became a household term. In fact, homologous recombination is the fundamental mechanism that allows for gene targeting in mice, a cornerstone of modern biomedical research. Scientists would insert a stabbed fragment of DNA into a cell with large regions of homology with a particular location in the genome. This new DNA would then be added to the chromosome by the HR machinery of the cell whereby a gene can be accurately knocked out or modified.
Although CRISPR-Cas9 is a programmable pair of molecular scissors that makes a very clean double-strand break at a targeted location, cellular endogenous repair mechanisms are used to introduce mutations into the DNA and alter the sequence. To achieve precise “gene editing” rather than error-prone “gene disruption,” scientists coax the cell to use the homologous recombination pathway. They accomplish this by offering a donor template of DNA with CRISPR equipment. In this template the desired edit is surrounded by homologous arms. As Cas9 cuts the DNA, the cell may employ this donor template through HR in order to insert the new sequence in a perfect way. The unique synthesis of the ease of targeting with CRISPR along with the high specificity of HR is leading genetic engineering into a new age of therapeutic possibilities.
Conclusion
Homologous recombination is far more than an obscure biological term; it is a cornerstone of life’s complexity and resilience. Whether it is a non-negotiable role of the master repair technician correcting the most lethal kind of DNA damage at a resolution of just a few molecules, or a creative role in the shuffling of the genome during meiosis, this is central to health and diversity, and evolution. Its essential nature is stressed by the dire outcomes of its breakdown that are best observed in the context of familial malignancies. Moreover, as we have now learned more about this ancient cellular pathway, we have now become genetic engineers ourselves, hijacking its precision in both research and medical applications. As we continue to unravel the complexities of homologous recombination, we unlock new potential for treating disease, understanding our own biology, and responsibly manipulating the very code of life. Next time you hear of some exciting advance in the treatment of cancer or in genome editing, recall the exquisite, essential cellular mechanism that so often facilitates it.